|
元 素
|
电 极 反 应
|
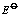 (V) (V)
|
|
Sc
|
Sc3++3e=Sc
|
-2.1
|
|
Se
|
Se+3e=Se2-
|
-0.92
|
|
Se+2H++2e=H2Se
|
-0.40
|
|
SeO42-+4H++2e=H2SeO3+H2O
|
+1.15
|
|
Si
|
Si+4H++4e=SiH4(g)
|
+0.10
|
|
SiF62-+4e=Si+6F-
|
-1.2
|
|
SiO2+4H++4e=Si+2H2O
|
-0.86
|
|
Sn
|
Sn2++2e=Sn
|
-0.14
|
|
Sn4++2e=Sn2+
|
+0.154
|
|
SnCl42-+2e=Sn+4Cl-
|
-0.19
|
|
SnCl52-+2e=SnCl42-+2Cl-
|
+0.14
|
|
HSnO2-+H2O+2e=Sn+3OH-
|
-0.91
|
|
Sn(OH)62-+2e=HSnO2-+H2O+3OH-
|
-0.93
|
|
Sr
|
Sr2++2e=Sr
|
-2.89
|
|
Ti
|
Ti2++2e=Ti
|
-1.63
|
|
Ti3++e=Ti2+
|
-0.37
|
|
Ti4++e=Ti3+
|
-0.09
|
|
Ti 62-+4e=Ti +6F-
|
-1.24
|
|
TiO2++2H++e=Ti3++H2O
|
+0.1
|
|
TiO2+4H++4e=Ti+H2O
|
-0.86
|
|
Tl
|
Tl++e=Tl
|
-0.336
|
|
Tl3++2e=Tl+
|
+1.26
|
|
V
|
V2++2e=V
|
约-1.2
|
|
V3++e=V2+
|
-0.255
|
|
VO2++2H++e=V3++H2O
|
+0.34
|
|
VO2-+2H++e=VO2++H2O
|
+0.999
|
|
VO2-+4H++5e=V+4H2O
|
0.25
|
|
W
|
W(CN)32-+e=W(CN)31-
|
+0.46
|
|
WO2+4H++4e=W+2H2O
|
-0.12
|
|
2WO3+2H++2e=W2O5+H2O
|
-0.03
|
|
WO3+6H++6e=W+3H2O
|
-0.09
|
|
WO42-+4H2O+6e=W+8OH-
|
-1.01
|
|
W2O5+2H++2e=2WO2+H2O
|
-0.04
|
|
Zn
|
Zn2++2e=Zn
|
-0.7623
|
|
Zn(CN)42-+2e=Zn+CN-
|
-1.26
|
|
Zn(NH3)42++2e=Zn+4NH3
|
-1.04
|
|
Zn(CN)22-+2H2O+2e=Zn+4OH-
|
-1.126
|
摘自 John A.Dean;Lange’s Hendbook of
Chemistry,13th Ed.(1985)